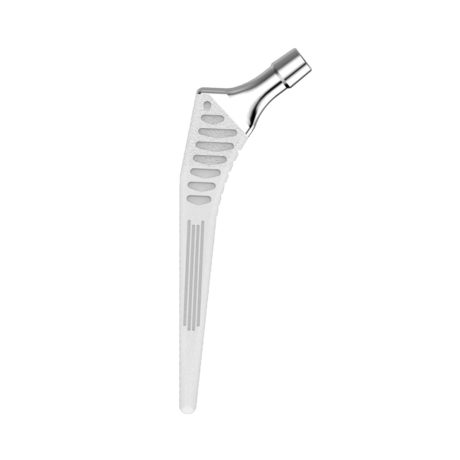
SAGITTA EVL R Stem
The SAGITTA EVL R stem is an anatomical femoral implant for revision surgery designed for prosthesis revision with or without a femoral flap. The stem is destined to a cementless fixation and its fixation is ensured by the HA coating.
The stem is anatomical to match the curvature of the femur and is available for right and left THA revisions. It is available in three lengths (180, 250 and 325 mm) with up to 5 diameters per size (12, 13, 14, 15 & 16).
Distal locking is provided by two locking screws, ensuring primery vertical and rotational stability.
Characteristics and Materials
Charactéristics:
- The SAGITTA EVL R stem has oval metaphyseal filling to ensure primary anchoring
- Anatomical stem available in right and left side versions
- CCD angle: 133°
- Two holes in the distal portion to accommodate two distal locking screws. The locking screws have a 5 mm diameter and are available in lengths of 25 to 65 mm
- The stem collar has a 11/13 (4°’) taper which needs to be used with SERF “SI” or “SD” heads with the same 11/13 taper
Materials:
- The SAGITTA EVL R stem is made from titanium alloy (TA6V) with an HA coating,
- The pins are also made from titanium alloy (TA6V),
- Femoral heads: “SI” made from stainless steel and “SD” made from Biolox® delta ceramic.
Instrumentation
A general kit and an additional tray according to the operated side are required to insert the SAGITTA EVL R stem.
Indications
Sagitta EVL R stems, (Sagitta REC) stems and optional associated locking screws are indicated for:
- revision of previous arthroplasty failure for any reasons ;
- reconstruction of previous arthroplasty failure for any reasons that need bone graft.
Note: etiologies /reasons of arthroplasty failure could be loosening, fracture /periprosthetic fracture, migration, polyethylene wear, dislocation, osteolysis and other reasons reported in literature.
Contraindications :
- patient with a known allergy to one of the prosthesis components ;
- any contraindications related to surgery or anesthesia.