NOVAE® Acetabular Cups
The Novae® range is based on the Dual Mobility concept invented in 1975 by Professor Gilles BOUSQUET (Saint-Etienne University Hospital) and Mr André RAMBERT, founder of SERF.
Dual Mobility is the combination of two principles in orthopaedics:
- Sir Charnley’s low friction concept: use of thick polyethylene with a small 22.2 mm head to reduce wear.
- The McKee-Farrar concept: use of a large head to reduce the risk of prosthesis dislocation and instability.
The Dual Mobility design is made of two bearing surfaces :
- Small articulation (or first mobility): articulation of the prosthesis head in the insert.
- Large articulation (or second mobility): articulation of the insert in the cup.
Characteristics and materials
The Novae® range includes 8 implants :
Cups and accessories:
- Novae® SunFit TH is intended for cementless fixation due to its equatorial press-fit and dual coating.
- Novae® E TH has two anchoring pegs and one additional fixation point for VCI screw to complete bone anchoring.
- Novae® Coptos TH has two anchoring pegs and four additional fixation points for VCI screws to complete bone anchoring.
- Novae® Stick must be cemented to the bone and may be combined with the Novae® K E reinforcement plate.
- Novae® K E reinforcement plate secured with fixing VCI screws.
Liners :
- Novae® CI E is intended for use with NOVAE cups.
- Novae® XPEO-E is intended for use with Novae® cups.
Novae® implants are made from the materials listed below :
- Cementless cup: Stainless steel + dual coating made of titanium spray and HA.
- Cemented cup and fixing screw: Stainless steel.
- Pegs : Alumina-coated stainless steel (uncoated stainless steel for US market)
- Novae® K E cross plate: Stainless steel and PMMA
- CI E liner: UHMWPE
- XPEO-E : Vitamin E-stabilized highly crosslinked UHMWPE
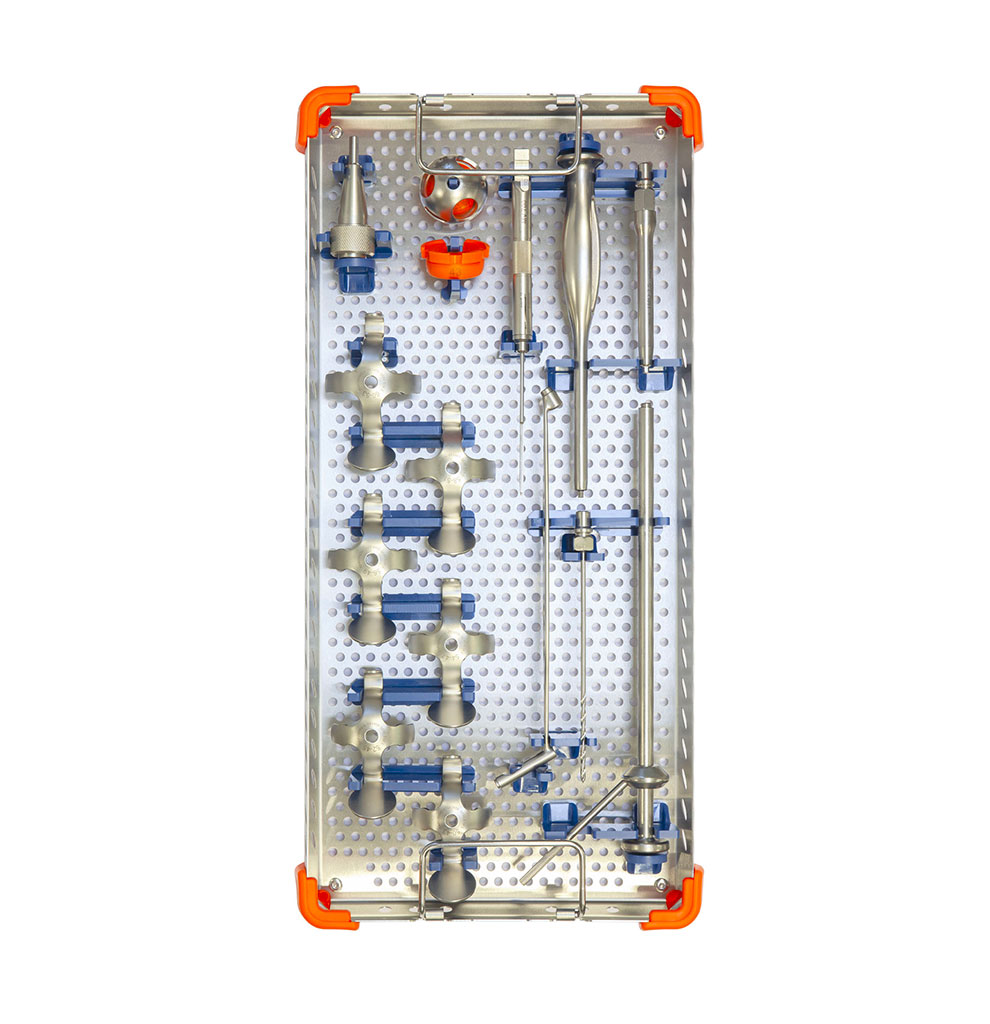
Instruments
- One instrumentation set common for all Novae® acetabular cups.
- Additional set for pegs and screw preparation for Novae® E TH and Coptos TH.
- Specific instrumentation for the Novae® K E cross plate.
Indications outside US
Novae® cups are particularly indicated for patients with a high risk of dislocation and for patients over 65 years.
Indications of the Novae® cup range differ according to the implant :
Novae® Sunfit TH :
Acetabular cups combining a “Sunfit TH” and a “CI E” or “XPEO-E” liner are intended for the treatment of the following conditions :
- Osteoarthritis of the hip, fractures of the femoral neck, osteonecrosis,
- Surgical revision (chronic dislocation, loosening, etc.) if the bone reconstruction allows, Paprosky type I, IIA and IIC bone defects.
Novae® E TH :
Acetabular cups combining a “Novae® E TH”, a “CI E” or “XPEO-E” liner and VCI screws are intended for the treatment of the following conditions :
- Osteoarthritis of the hip, osteonecrosis,
- Surgical revision (including recurrent dislocation and loosening) if the bone reconstruction allows, Paprosky type I, IIA and IIC bone defects.
Novae® Coptos TH :
Acetabular cups combining a “Novae® Coptos TH”, a “CI E” or “XPEO-E” liner and VCI screws are intended for the treatment of the following conditions :
- Surgical revision (including recurrent dislocation and loosening) if the bone reconstruction allows, with Paprosky type IIA, IIB, IIC and IIIA.
Novae® Stick :
Acetabular cups combining a Novae® Stick cup and a liner (CI E or XPEO-E) are intended for treatment of the following conditions :
- osteoarthritis of the hip,
- fractures of the femoral neck,
- osteonecrosis,
- surgical revision (including recurrent dislocation and loosening) if the bone reconstruction allows,
- with Paprosky type IIIA to type IIIB,
- periacetabular metastatic bone disease.
Novae® K E :
Acetabular cups combining a Novae® Stick, a Novae® K E reinforcement plate, VCI screws and a liner “CI E” or “XPEO-E”* are intended for the treatment of the following conditions :
- surgical revision (including recurrent dislocation and loosening) if the bone reconstruction allows, with Paprosky type IIIA to type IIIB ;
- periacetabular metastatic bone disease.
Contraindications of the Novae® range are as follows :
Contraindications relating to use of Novae® dual mobility cups and liners are local or systemic acute or chronic conditions (heart disease, uncompensated diabetes, regular haemodialysis, impairment of the immune system, etc.).
Indications for US market
The Novae® Dual Mobility System is indicated for total hip replacement, which includes:
- osteoarthritis,
- femoral neck fracture,
- dislocation risk,
- osteonecrosis of the femoral head,
- revision procedures where other treatments or devices have failed and if bone reconstruction so permits.
Sunfit TH, Novae® E TH, and Coptos TH acetabular cups are intended for press-fit use and Novae® Stick acetabular cup is intended for cemented use.
Contraindications relating to use of Novae® dual mobility cups and liners are local or systemic acute or chronic conditions (heart disease, uncompensated diabetes, regular hemodialysis, impairment of the immune system, etc.).