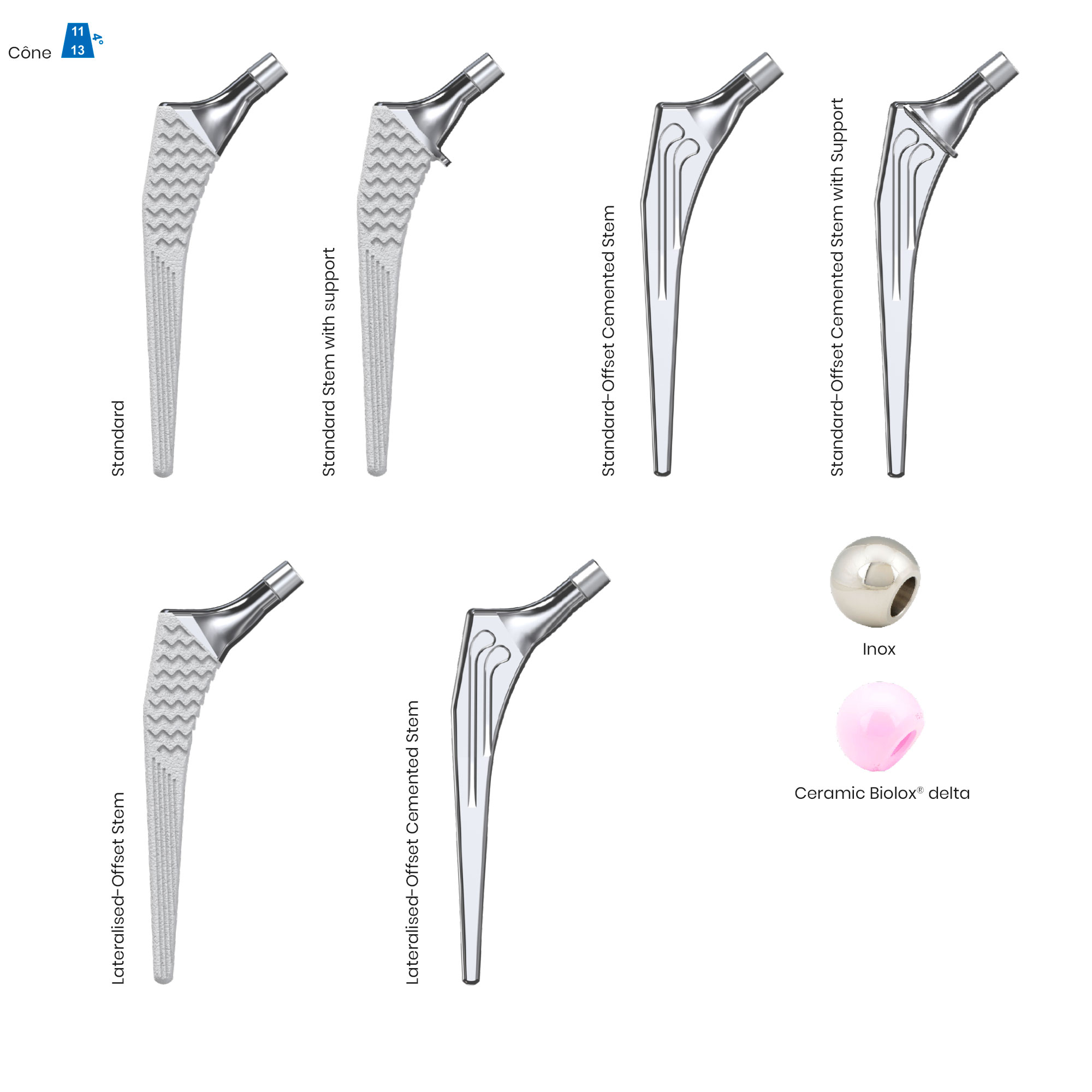
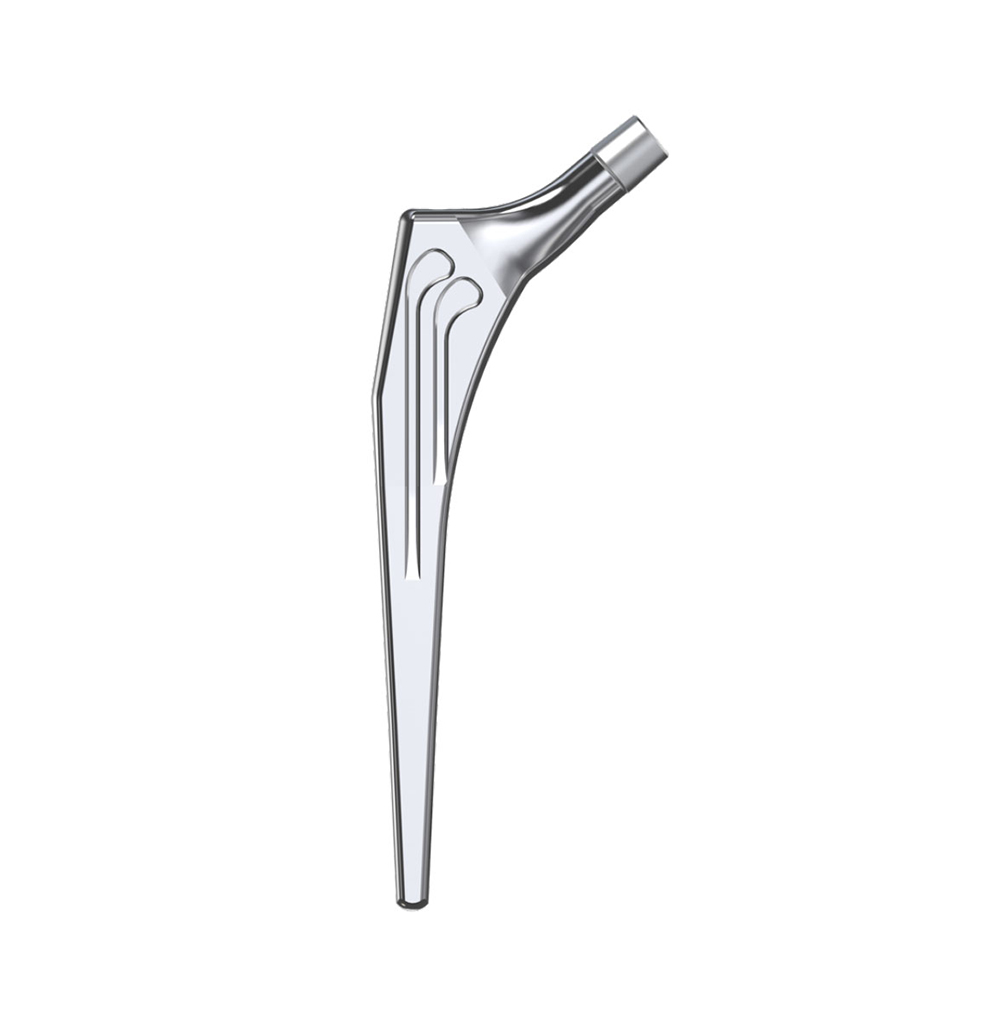
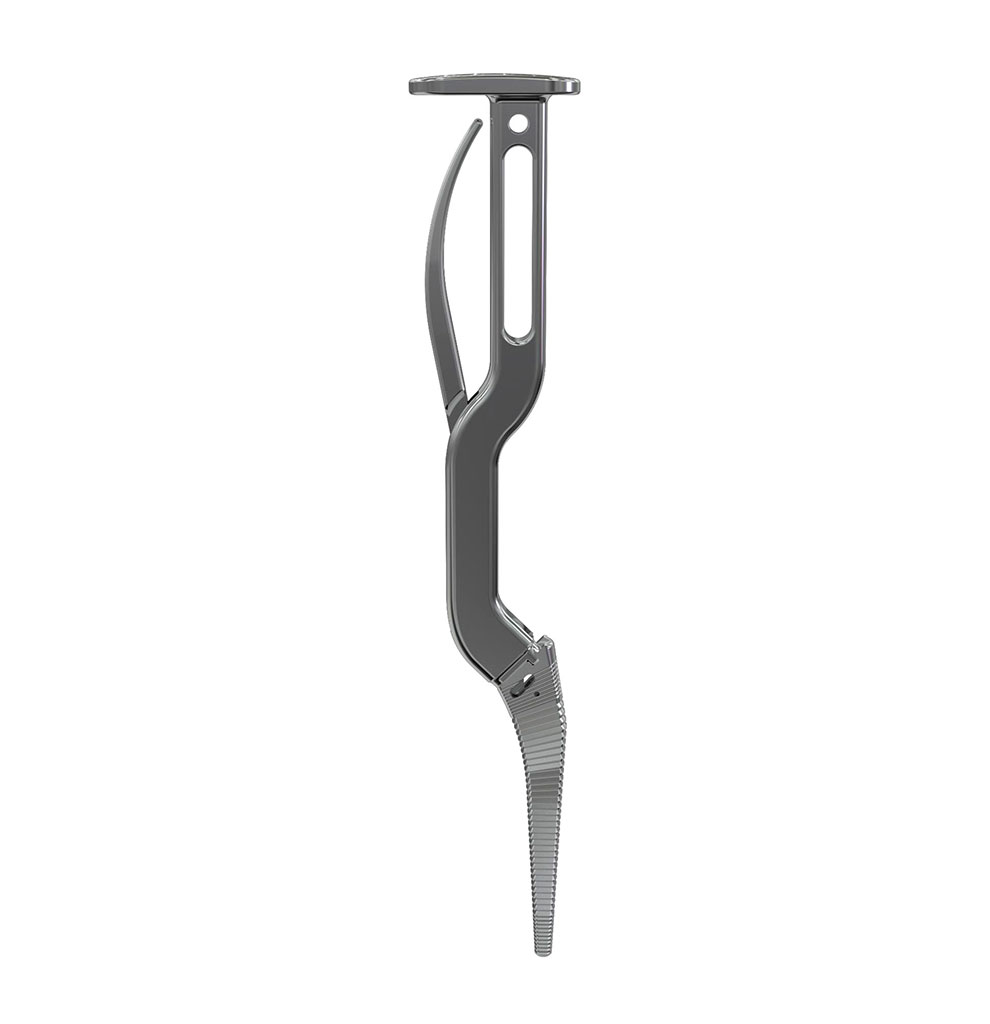
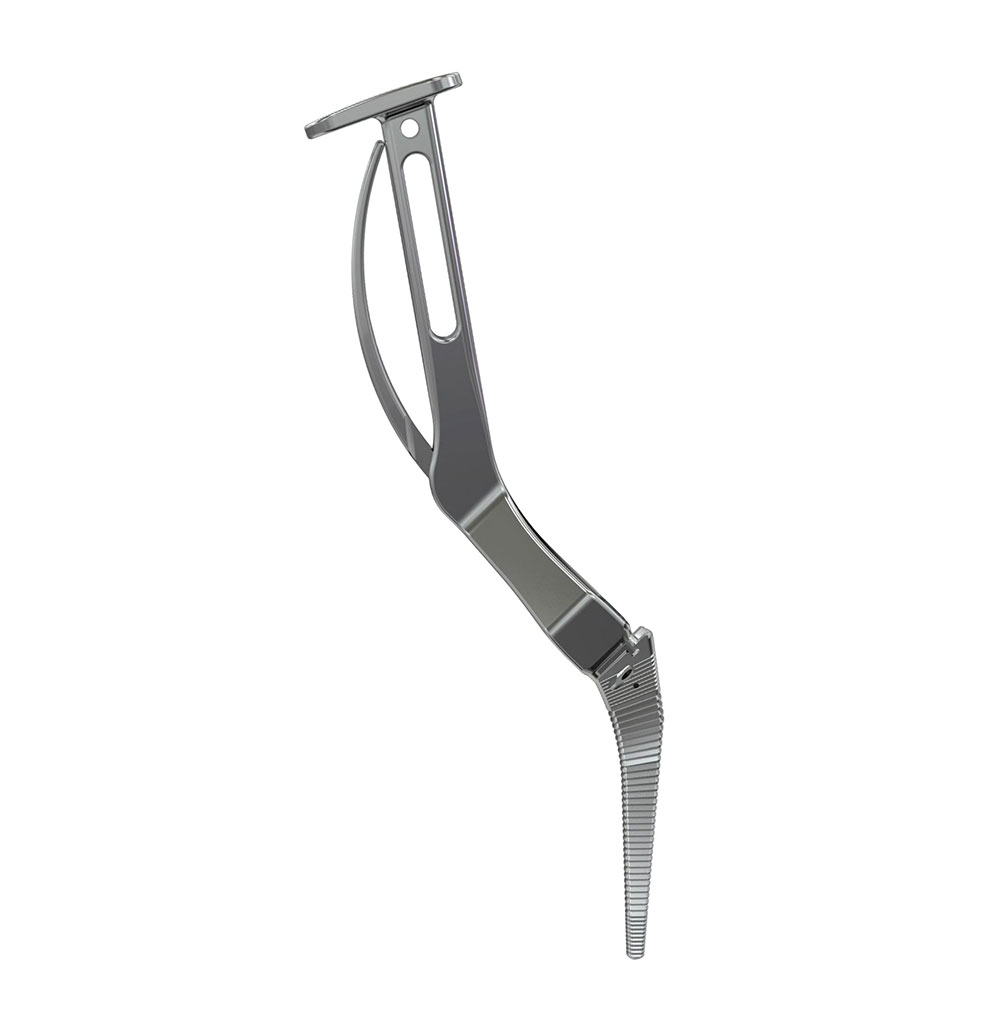
Libra® Stems
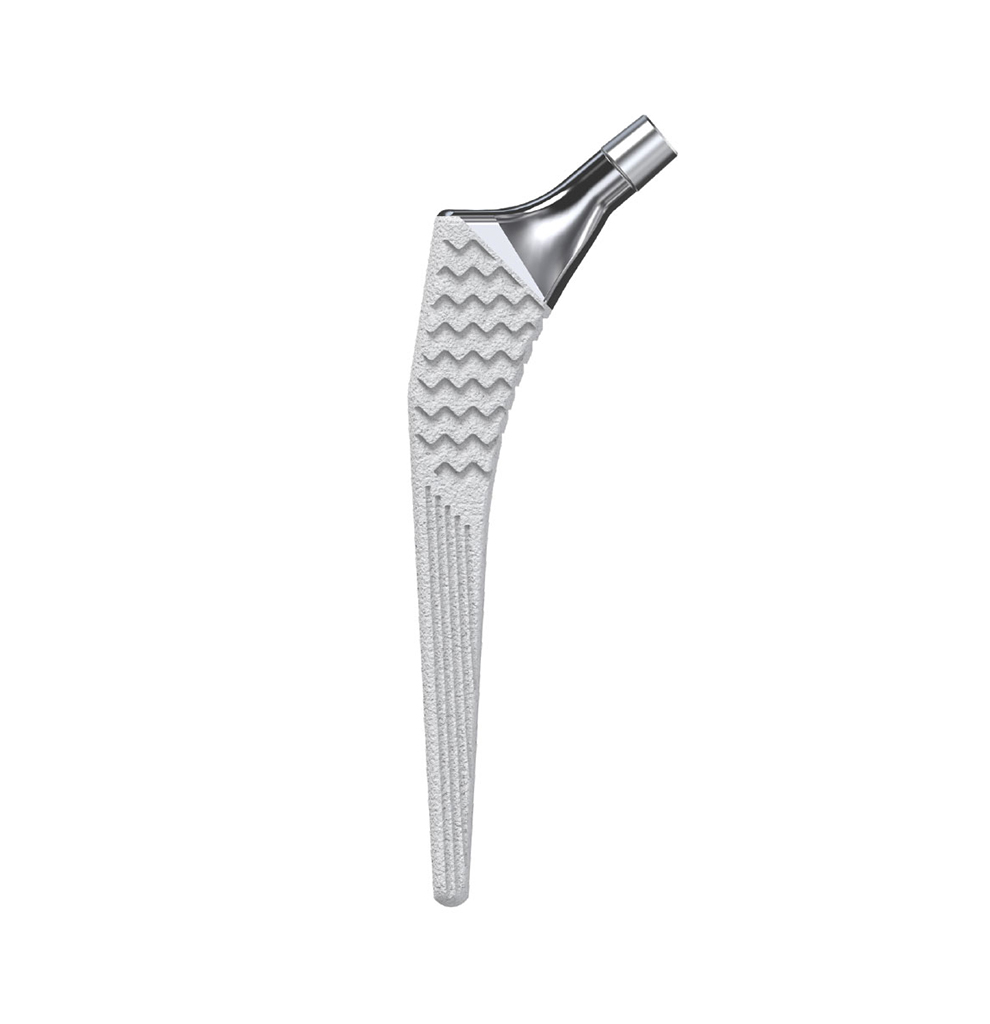
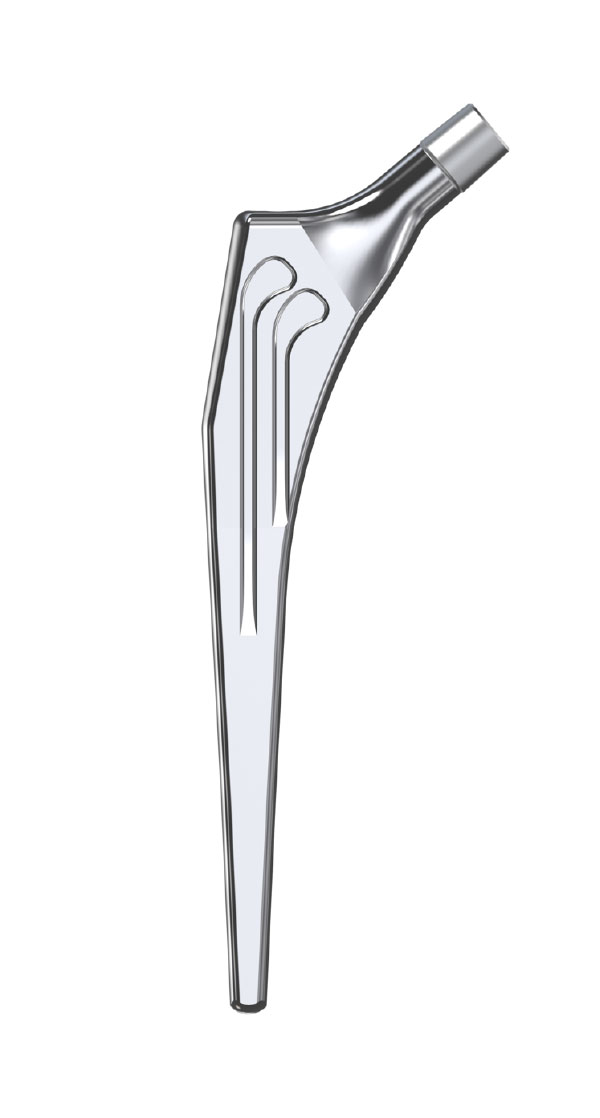
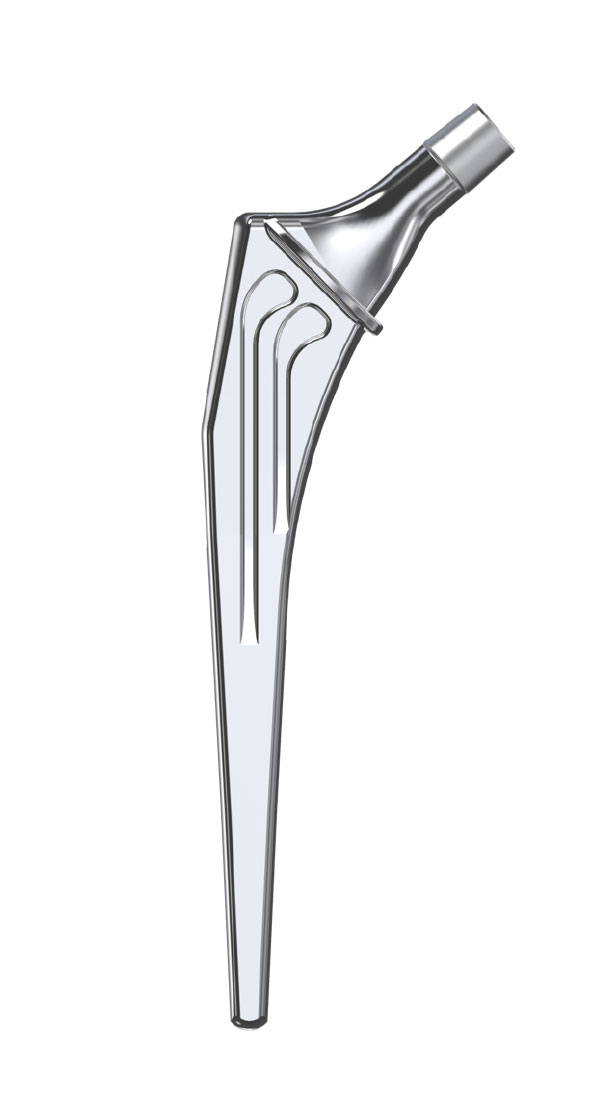
The Libra® range of implant is composed by self locking stems dedicated to primary total hip arthroplasty or revision surgery. The stems have a 11/13 (4°) taper.
Characteristics and materials
Characteristics :
- Standard or lateralised, collared or collarless version.
- Cemented or cementless versions, with or without support.
- CCD angle of 130°.
- A 11/13 (4°) taper which needs to be combined with SERF “SI” or “SD” heads with the same 11/13 taper.
Materials :
- Cementless stems: titanium alloy and HAP
- Cemented stems: fully polished high-nitrogen stainless steel.
- Femoral heads: “SI” made from stainless steel or “SD” made from Biolox® delta ceramic.
Instruments
- An unique instrumentation
- A wide choice of broach handles for the different approaches : posterior, posterolateral, anterior and anterolateral.
Indications
The Libra® stem range is indicated in the following cases:
- Primary or secondary arthrosis
- Fractures of the proximal femur
- Revision of a previous surgery, including hip arthroplasty
- For revision of surgery other than hip arthroplasty, the implantation of the femoral stem must not interfere with the equipment that is not removed, if applicable
- For hip arthroplasty revision surgery, the implanted femoral stem must be compatible with the equipment that has not been removed if applicable
- Only for a cementless femoral stem implantation, these indications are appropriate according to the following classifications: type 1, 2A and 2B of the Paprosky classification.
Contraindications of the Libra® stem range are as follows:
- Localized or systemic acute or chronic infection
- Skeletal immaturity
- Pregnant or nursing women
- Known allergy to one of the prosthesis components
- Severe muscular, neurological, or vascular deficiency affecting the concerned limb
- Bone degradation or bone loss, or poor bone quality likely to affect the stability of the implant
- Local bone tumors
- Obesity.